Case study
Targeted reduction of process-related impurities
We shortened our process optimization by at least one year - and saved $1 million
This client worked on the purification process of an E. coli expressed protein. When discovering too high a level of Host Cell Proteins (HCPs), they did not know how to efficiently optimize their process development. LC-MS-based HCP analysis enabled them to document individual process impurities and intelligently optimize the purification process within a week.
The client is a clinical-stage biotech company with European headquarters and offices in the USA. They develop next-generation biopharmaceuticals in the form of antibody mimetics.
Our experts performed HCP analysis by SWATH mass spectrometry on six different process samples. In the results, the total HCP amount (ng HCP/mg drug substance) was reported, along with the amount of each HCP in every sample.
The table shows that the data package also included physiochemical parameters such as molecular weight and pI. In addition, the client received the database names and accession numbers of all the identified E. coli proteins. Subsequently, the client can use the information for risk assessment of each protein.
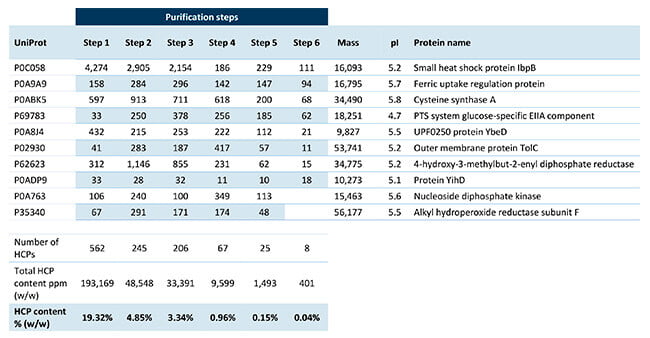
The 10 most abundant Host Cell Proteins in the six product purification samples.
Reducing HCP to acceptance criteria
The detailed HCP outline of the entire purification process allowed the client to actively modify the process steps. Consequently, they were able to reduce HCPs to a level below acceptance criteria in only one week. They now no longer have to work in the blind and optimize various steps without any HCP reduction.
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.