Case study
Comparing viral protein quantities in AAV batches and drug products
"We were on the lookout for problematic Sf9 and AcMNPV host cell proteins"
What would it mean for your AAV manufacturing if your HCP assay could quantify proteins from both the cell line and its expression vector system? In this case, such an assay enabled a gene therapy CMO to compare and document the batch consistency. In addition, they were able to locate any differences in the AAV products.
The CMO manufactures adeno-associated virus (AAV) based products for treating rare genetic diseases. They needed analytics to provide detailed information about the viral and residual proteins present. The results were intended to investigate between-run reproducibility.
However, the product’s complex manufacturing process, involving infecting Spodoptera frugiperda (SF9) cells with a baculovirus – AcMNPV – to generate AAV5-based therapies, required a rigorous approach.
We started by analyzing the viral protein amount in the AAV batches. Using SWATH LC-MS, we identified and quantified the individual viral proteins. Figure 1 below compares the total viral protein amount in three sets of two AAV product variants:
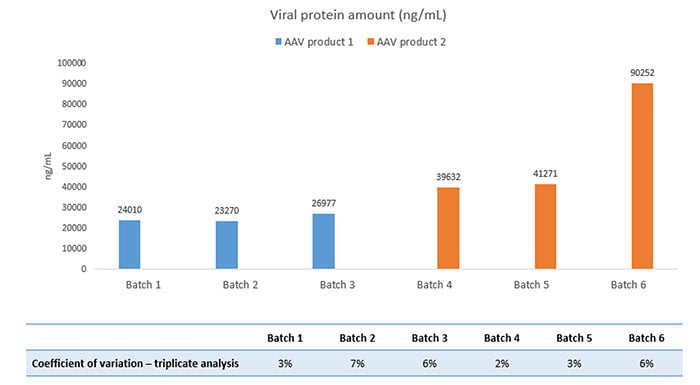
Figure 1
Interestingly, while the overall viral content was consistent in AAV product 1, we saw that batch 6 of AAV product 2 varied considerably compared to batch 4 and 5. Therefore, the client began investigating batch 6 in more detail, resulting in an optimized manufacturing process. The result was a process scale-up to 2000L batches without significant differences in the total viral amount produced.
Identification of problematic Sf9 and AcMNPV host cell proteins
We used the same LC-MS assay for analyzing residual proteins. We did not need to re-run the samples and re-analyze the data to look for specific residual proteins. Thus, the process merely requires adding the proteins of interest to the mass spectrometry database.
Next, the team compared the identified proteins to those found in other AAV products and common biologics based on recombinant proteins and monoclonal antibodies. Table 2 shows that the comparison detected a peptidylprolyl isomerase (often seen in CHO-produced biologics). We also found ubiquitin (a degradation-pathway protein) in the baculovirus, AcMNPV, and the Sf9 cells.
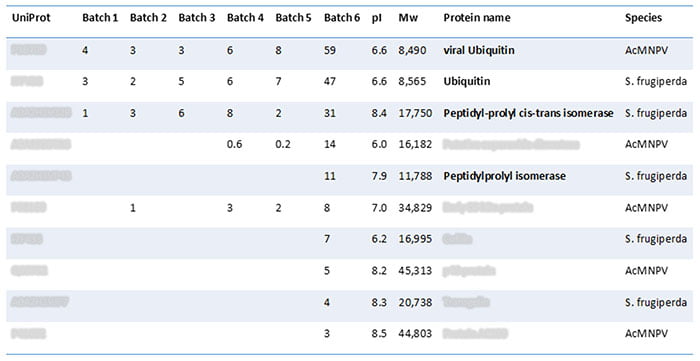
Table 1
The client included the analysis data in the regulatory dossier. They also plan to set up specific assays for continued monitoring of the proteins of concern in the two products.
Find out more about the gene therapy product analysis here
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.