Case story
FDA approves IND using LC-MS-based Host Cell Protein assay
The first example of regulatory authorities approving an IND without HCP-ELISA data
Actinobac Biomed Inc recently received FDA approval for their Investigational New Drug Application (IND) using only mass spectrometry data to monitor host cell proteins. Thus, they did not have to spend 1-2 years setting up a process-specific HCP-ELISA – that may not even work.
This US-based client develops a biopharmaceutical product from an unusual cell line, for which there is no commercial HCP-ELISA kit available. Therefore, they could either create a process-specific ELISA from scratch or use an orthogonal method not reliant on anti-HCP antibodies.
They asked Alphalyse to develop a mass spectrometry-based host cell protein (HCP) assay, which they could use for process optimization. It provided them with a list of the HCPs in their drug product, so the client decided to qualify the assay and include the data in their IND application.
Qualification parameters
The assay qualification included the following parameters:
- Specificity
- Limit of detection (LOD)
- The lower limit of quantitation (LLOQ)
- Linearity and calibration curves
- Accuracy
- Precision
All parameters fell within the acceptance criteria, and the qualification was thus successful.
In Table 1, you can see the intermediate precision across the triplicate analysis. We also analyzed the amount of each of the 19 most abundant HCPs and the total amount of HCP, as shown in Table 2.
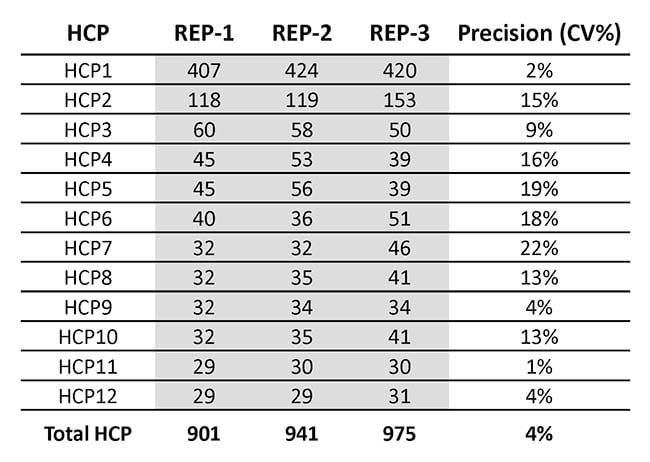
Table 1: Anonymized quantification of Host Cell Proteins in a triplicate analysis of drug substance
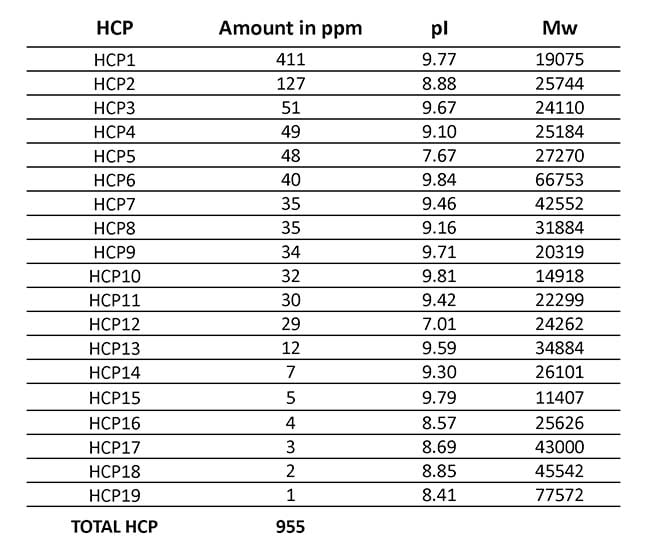
Table 2: Average amount of 19 most abundant HCPs present in the final drug substance
After the successful qualification, the client submitted their IND and received FDA approval. To our knowledge, this is the first example of authorities accepting an IND containing only HCP data from a mass spectrometry assay – without also requesting data from ELISA experiments.
If you are interested in learning more, we also recommend watching this webinar on how we are moving LC-MS HCP analysis into a GMP environment

Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.