Case study
Purified mAb with difficult-to-remove HCP impurity (ubiquitin)
How a detailed analysis enabled risk assessment of Host Cell Proteins in a mAb DS
A clinical-stage biopharmaceutical company wanted a thorough analysis of their process’ ability to remove Host Cell Proteins (HCPs) from their CHO-expressed monoclonal antibody (mAb).
Even though their HCP-ELISA reported no detectable HCPs in the purified drug substance, LC-MS analysis identified one HCP impurity, as can be seen in the table below.
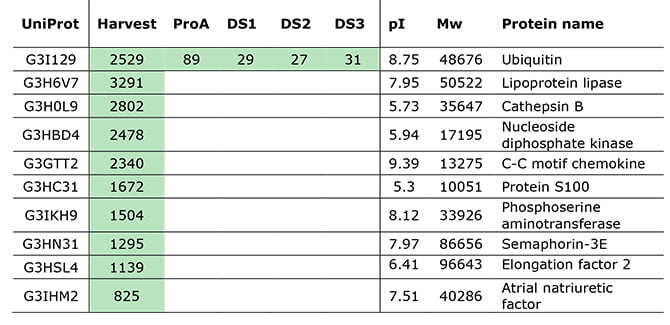
HCP quantifications (ppm) of the top 10 HCPs, quantified by MS signal in a sample prepared with standards. Green indicates levels above LLOQ.
The specific HCP detected by LC-MS – but missed by ELISA – was ubiquitin.
Ubiquitin is a protein that sticks to protein substrates through ubiquitination, and it is a prevalent posttranslational modification. Ubiquitination marks other proteins for degradation via the proteasome pathway, and it can thus affect protein activity and cellular location.
How the client used the LC-MS data
The client wanted to do a risk assessment of discovered and documented ubiquitin in their drug substance with the LC-MS data. During their risk assessment, they found that you also find this common HCP in many other mAb products.
It is still uncertain if they can or should reduce the ubiquitin level further. However, now the client knows the absolute quantity of this specific HCP and can monitor the ubiquitin levels from batch to batch.
Talk to us
Whatever protein-related challenge or question you may have, we would love to help. Our experts can help you decide on the best analytical approach for your project by email or online meeting - providing advice without obligation.